Describe the Following Compounds Using Grignard Reagents
H OH- pH pOH. Use ANY needed reagents to effect the following transformation.
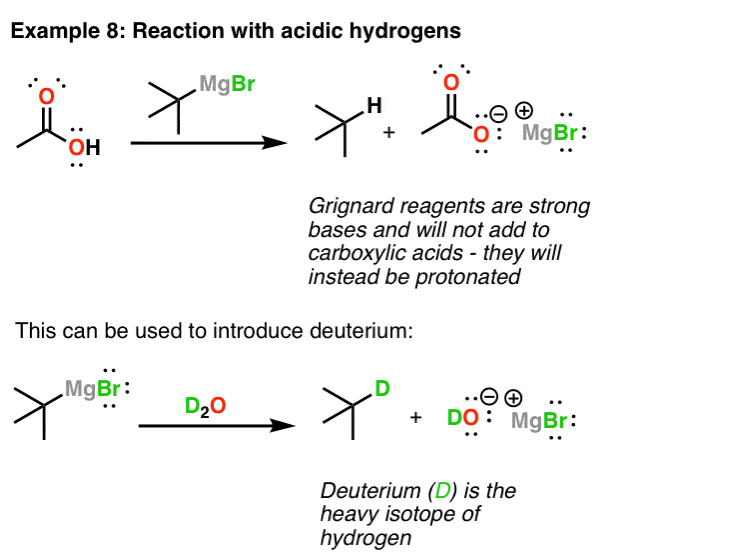
Grignard Reagents In Organic Chemistry Master Organic Chemistry
Water is dissociated at 25C.

. So protic solvents are not suitable for them. Grignard reagents RMgX highly react with proton. Organic compound transformation reactions.
Click to see the answer. A What is a difference between traditional Williamson ether synthesis and reaction A. Given- Concentration of barium hydroxide.
Fill in the table. Solution for Describe in detail the N-F bond in terms of the relevant electronegativities and polarities. The drying process itself is slightly exothermic ΔH for the process is negative but the entropy decreases.
Draw the product draw the A. How many milliliters of an aqueous solution of 0124 M barium hydroxide is needed to obtain 127 A. The Gibbs free energy for the following reaction Na 2 SO 4 10 H 2 O ---- Na 2 SO 4 10 H 2 O can be described by ΔG ΔH - TDS -778 kJmol - T -261 JmolK Based on this equation it can be estimated that the reaction reverses at 298 K or 25 o C ΔG 0.

In The Following Practice Exercise We Will Determine The Major Product Of A Grignard Reagent Wit Study Chemistry Organic Chemistry Organic Chemistry Reactions
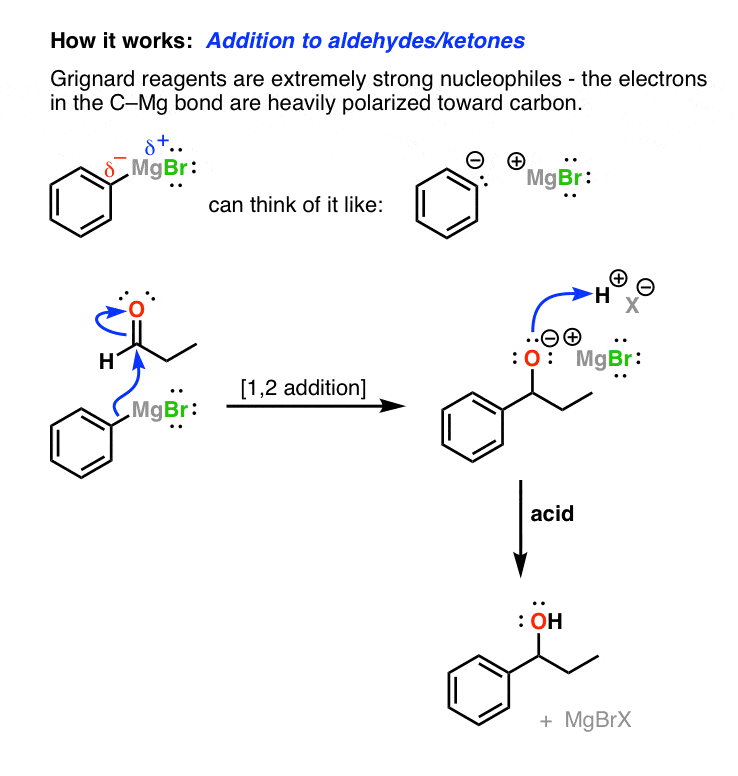
Grignard Reagents In Organic Chemistry Master Organic Chemistry

Reactions Of Aromatic Compounds Organic Chemistry Teaching Chemistry Organic Chemistry Books
No comments for "Describe the Following Compounds Using Grignard Reagents"
Post a Comment